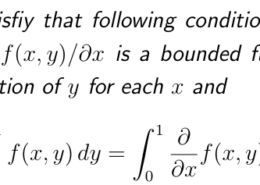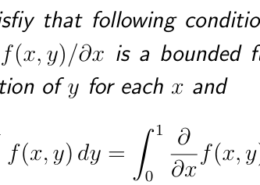Be sure to answer all parts The C-H bond in acetone, (CHj)C, has a pKa of 19.2. Draw two resonance structures for its conjugate base. Then, explain why acetone is much more acidic than propane, CH3CH2CH3 (pKa – 50 Resonance structures: draw structure draw structure (negative charge on (negatise charge an o) (negative charge on C) (negative charge on O)
ReportQuestion
Be sure to answer all parts The C-H bond in acetone, (CHj)C, has a pKa of 19.2. Draw two resonance structures for its conjugate base. Then, explain why acetone is much more acidic than propane, CH3CH2CH3 (pKa - 50 Resonance ...