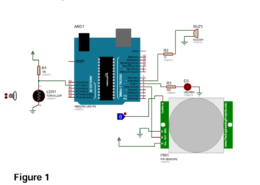
Problem 6 The switch in the circuit has been in position a for a long time. At t = 0 it moves to position b. Calculate i(t) for allt > 0. a t=0 w 632 30 v 12 v © {39 +2F Problem 7 Find v(t) fort 0 in the circuit below. 0.5 H non 3923 + Alle 24 V 20 V
ReportQuestion

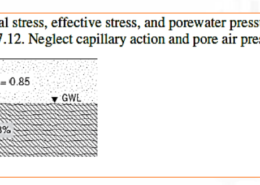
![The decomposition of hydrogen peroxide (H2O2) produces water and oxygen in a first-order reaction. The concentration of hydrogen peroxide, 𝐶, at any time, 𝑡, is thus given by: 𝐶 = 𝐶0𝑒 −𝑘𝑡 where: 𝐶 is the concentration of Hydrogen Peroxide in mol L −1 at time 𝑡, 𝐶0 is the initial concentration in mol L −1 , 𝑡 is the time elapsed in s, and 𝑘 is a rate constant in s −1 . In an experiment the concentration is measured to be 0.225 ± 0.005 mol L −1 after 20.0 ± 0.5 s. It is found that 𝐶0 = 0.25 mol L −1 with negligible uncertainty. a) Show that the equation can be linearly transformed into the form 𝑦 = 𝛽̂ 0 + 𝛽̂ 1𝑥 and define 𝑦, 𝛽̂ 0, 𝛽̂ 1, and 𝑥. [4 marks] b) Determine the estimate and uncertainty for the rate constant, 𝑘, and report both to an appropriate number of significant figures. [7 marks] c) A proposal is made to change the measurement devices in order to reduce the uncertainty in the rate constant, 𝑘. It is possible to reduce either the uncertainty in 𝐶0 to ±0.004 mol L −1 or the uncertainty in 𝑡 to ± 0.4 s −1 . Which change should be made? Justify your answer. [2 marks] d) A counter proposal suggests that the error in 𝑘 can be reduced without changing the measurement devices as suggested in part c). Instead, a change to the experimental procedure can be made by reading the concentration, 𝐶, at a time different to 20 s. Is the recommendation valid? If so, should the reading be made earlier or later than 20 s to reduce the uncertainty in k? Justify your answer and clearly state any limitations. (Max 100 words)](https://numengineering.com/wp-content/uploads/2024/12/Screenshot-2024-12-08-171619-260x185.png)