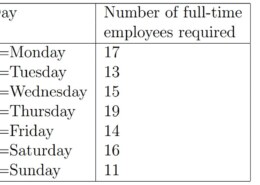
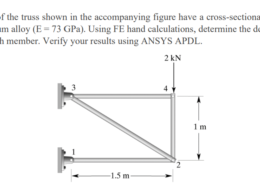
The members of the truss shown in the accompanying figure have a cross-sectional area of 15 cm2 and are made of aluminum alloy (E = 73 GPa). Using FE hand calculations, determine the deflection of each joint and the stress in each member. Verify your results using ANSYS APDL
ReportQuestion
![A pharmaceutical crystalline material containing 15% by weight H2O must be dried to 7% H2O in a recycle air dryer. The fresh air contains 0.01 kg H2O/kg dry air and the air leaving the dryer contains 0.1 kg H2O kg/kg dry air. The air entering the dryer contains 0.03 kg H2O/kg dry air. a) Draw a schematic diagram of the process. [2 marks] b) Calculate the weight of water removed and the weight of fresh air supplied per 10 kg of wet feed [4 marks] c) Calculate the weight of recycled air per 10 kg wet feed.](https://numengineering.com/wp-content/uploads/2024/12/Screenshot-2024-12-09-180635-260x185.png)
![Toluene is manufactured by the catalytic conversion of n-heptane according to the reaction: C7H16 → C6H5CH3 + 4H2 The n-heptane is fed to the reactor at a temperature of 400°C and the reaction goes to 20% completion. a) Choosing a suitable basis, calculate the number of moles of all components in the product stream. [2 marks] b) Write an energy balance for the system stating the references and any assumptions clearly. [2 marks] c) Calculate the standard heat of reaction at 25°C. [4 marks] d) Calculate how much heat has to be added or removed from the reactor to make it isothermal at 400°C. State any assumptions clearly. [4 marks] Data: Standard heats of formation at 1 atm and 25°C: n-heptane (l) -53.630 kcal/mole CO2 (g) -94.052 kcal/mole H2O (g) -57.798 kcal/mole Standard heat of combustion at 1atm and 25°C: Toluene (l) -934.5 kcal/mole Latent heat of vaporization at 1atm and 25°C: H2O 10.52 kcal/mole](https://numengineering.com/wp-content/uploads/2024/12/Screenshot-2024-12-09-164955-260x185.png)
![A pharmaceutical crystalline material containing 15% by weight H2O must be dried to 7% H2O in a recycle air dryer. The fresh air contains 0.01 kg H2O/kg dry air and the air leaving the dryer contains 0.1 kg H2O kg/kg dry air. The air entering the dryer contains 0.03 kg H2O/kg dry air. a) Draw a schematic diagram of the process. [2 marks] b) Calculate the weight of water removed and the weight of fresh air supplied per 10 kg of wet feed [4 marks] c) Calculate the weight of recycled air per 10 kg wet feed. [2 marks] d) What is the aim of the recycle? Briefly explain.](https://numengineering.com/wp-content/uploads/2024/12/Screenshot-2024-12-09-164906-260x185.png)
![A stream of saturated steam at 1 atm and a flow rate of 1150 kg/h is mixed with a second stream of superheated steam at 400°C in an adiabatic mixing. The resulting stream is a superheated steam at 300°C which is a feed to a heat exchanger. a) Draw a schematic diagram of the process. [2 marks] b) Write the energy balance for the system clearly stating the references and all assumptions. [2 marks] c) Calculate the amount of superheated steam at 300°C produced and the required volumetric flow rate of the 400°C steam. [4 marks] Additional data: Specific volume of superheated steam at 400°C is 3.11 m3 /kg For physical and thermodynamic data use Q1 Table 3 in the Additional Data Section.](https://numengineering.com/wp-content/uploads/2024/12/Screenshot-2024-12-09-164758-260x185.png)